RECENT CASE STUDIES
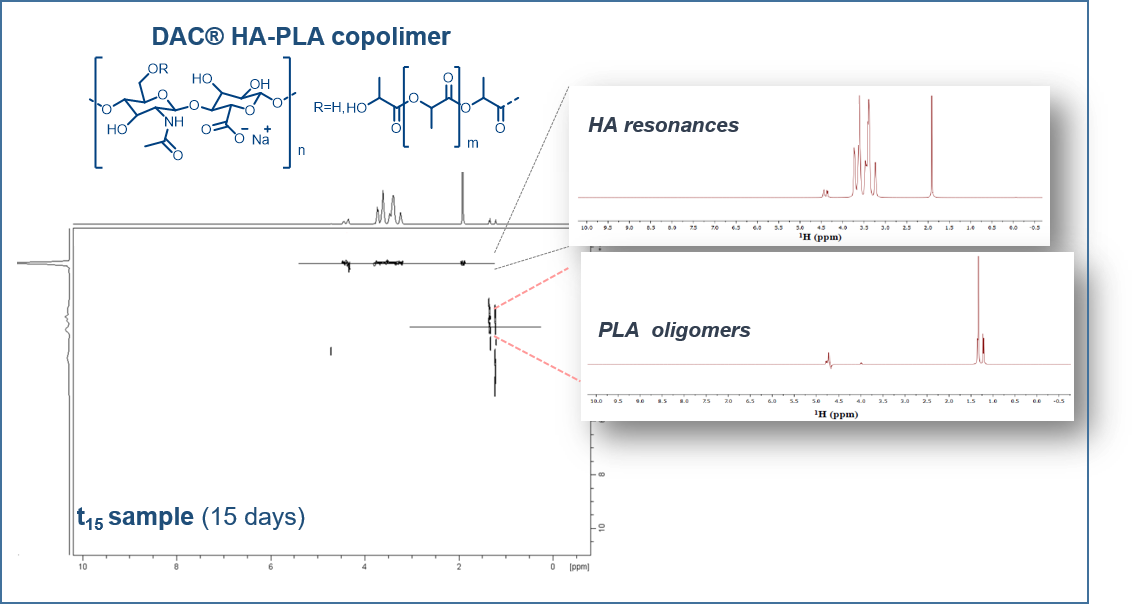
Stability Evaluation and Degradation Studies of DAC® Hyaluronic-Polylactide Based Hydrogel by DOSY NMR Spectroscopy
Abstract
The stability and the degradation of polymers in physiological conditions are very important issues in biomedical applications. The copolymer of hyaluronic acid and poly-D,L-lactic acid (made available in a product called DAC®) produces a hydrogel which retains the hydrophobic character of the poly-D,L-lactide sidechains and the hydrophilic character of a hyaluronic acid backbone. This hydrogel is a suitable device for the coating of orthopedic implants with structured surfaces. In fact, this gel creates a temporary barrier to bacterial adhesion by inhibiting colonization, thus preventing the formation of the biofilm and the onset of an infection. Reabsorbed in about 72 h after the implant, this hydrogel does not hinder bone growth processes. In the need to assess stability and degradation of both the hyaluronan backbone and of the polylactic chains along time and temperature, we identified NMR spectroscopy as a privileged technique for the characterization of the released species, and we applied diffusion-ordered NMR spectroscopy (DOSY-NMR) for the investigation of molecular weight dispersion. Our diffusion studies of DAC® in physiological conditions provided a full understanding of the product degradation by overcoming the limitations observed in applying classical chromatography approaches by gel permeation UV.
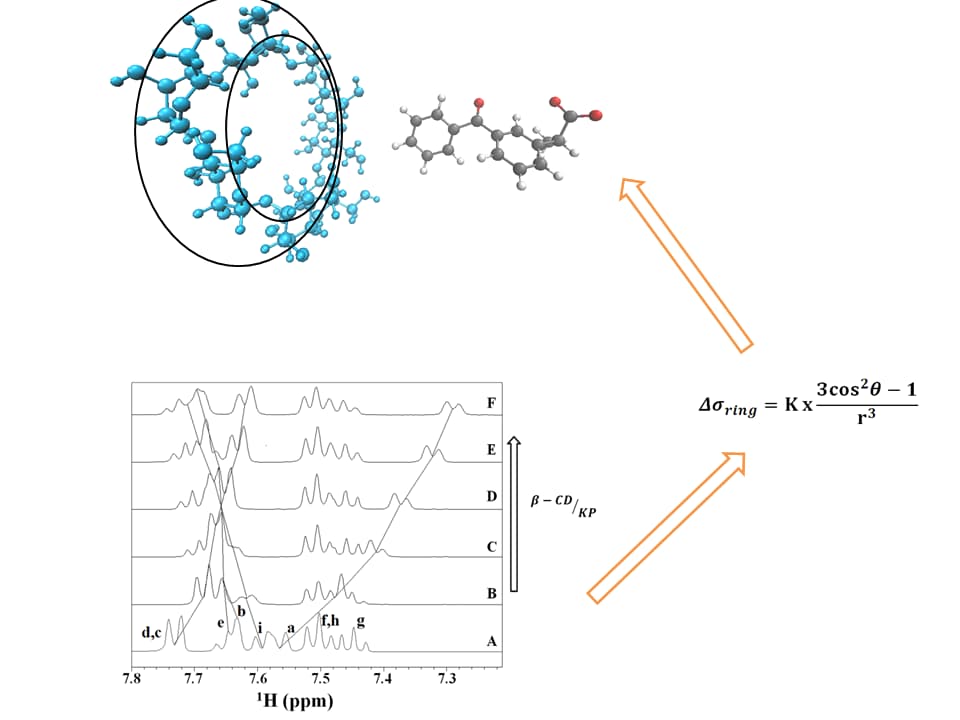
The Conformational Change in the Mechanism of Inclusion of Ketoprofen in β-Cyclodextrin: NMR Spectroscopy, Ab Initio Calculations, Molecular Dynamics Simulations and Photoreactivity
J. Phys. Chem. B
DOI: 10.1021/acs.jpcb.6b07913
Publication Date: 30 Sep 2016
Abstract
Inclusion of drugs in cyclodextrins (CDs) is a recognized tool for modifying several properties such as solubility, stability, bioavailability, and so on. The photoreactive behavior of the β-CD/ketoprofen (KP) complex upon UV exposure showed a significant increase in photodecarboxylation, whereas the secondary degradation products by hydroxylation of the benzophenone moiety were inhibited. The results may account for an improvement of KP photophysical properties upon inclusion, thus better fostering its topical use. To correlate the structural details of the inclusion with these results, an NMR spectroscopic study of KP upon inclusion in β-CD was performed. Effects of the magnetically anisotropic centers of KP, changing their orientations upon inclusion and giving chemical shift variations, were specifically correlated with the results of the molecular dynamic simulations and ab initio calculations. In the large variety of papers focusing on the structural analysis of β-CD complexes, this work represents one of the few examples in which a detailed analysis of these simultaneous upfield–downfield NMR shifts of the same aromatic molecule upon inclusion is reported. Interestingly, the results demonstrate that the observed upfield and downfield shifts upon inclusion are not related to any direct magnetic role of β-CD. The conformational change of KP upon the inclusion process consists of a slight reduction in the angle between the two phenyl rings and in a remarkable reduction in the mobility of the carboxyl group, the latter being one of the main contributions to the NMR resonance shifts. These structural details help in understanding the features of the inclusion complex and, eventually, the driving force for its formation.
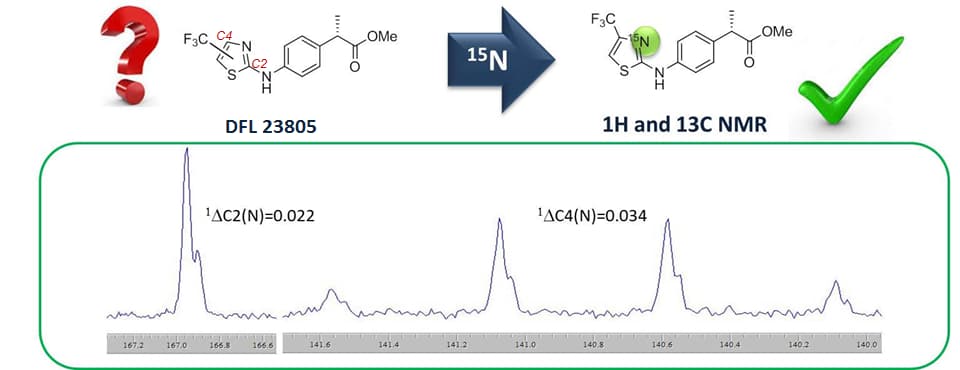
The 15N induced isotope shift as an effective tool for the structure elucidation of 2,4 and 2,5 di-substituted thiazoles
Tetrahedron Letters
DOI: 10.1016/j.tetlet.2015.05.084
Publication Date: 28 May 2015
Abstract
15N isotope effects have been used for structural elucidation of the di-substituted thiazole 1-DFL 23805, a novel and potent dual CXCR1/CXCR2 inhibitor, developed by Dompé within the MedChem program. The compound and its 15N isotopologue have been synthesized according to the same proprietary procedure. The 1H and 13C NMR spectra have been measured. The isotope effects on chemical shifts and on coupling constants have been studied in detail. The collected data highlight a new perspective on the elucidation of disubstituted thiazole system, as only very few reference data have been published so far. All the results are consistent with the 2,4 regioisomery assignment.